Ocean Beach Hospital administers first shots of covid-19 vaccine in Pacific County
Published 11:16 am Friday, December 18, 2020
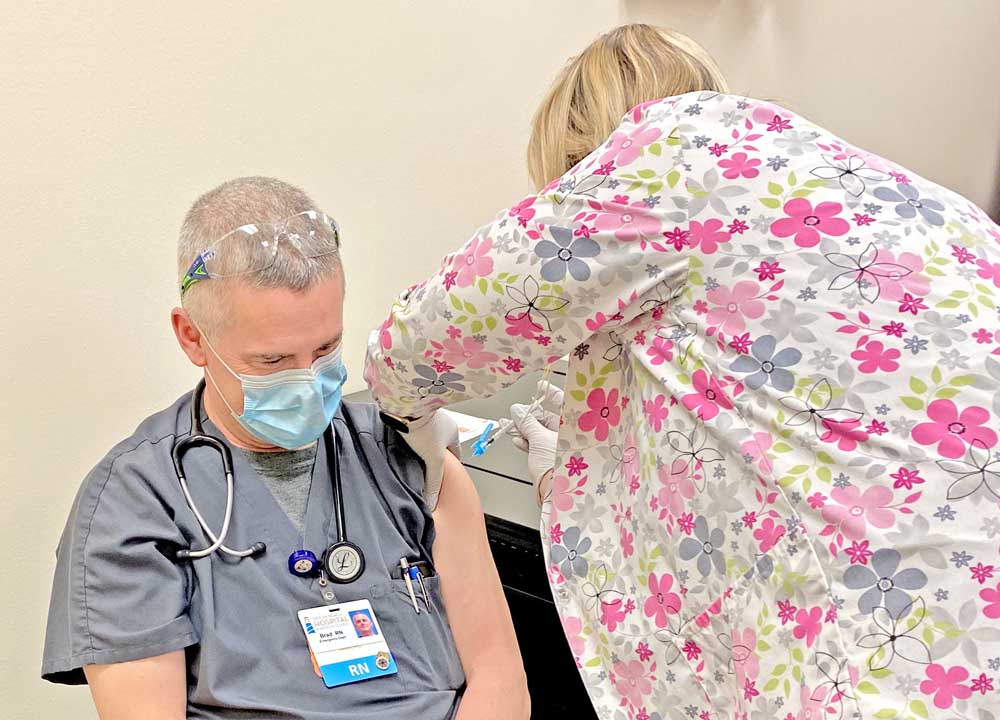
- Oeach Beach Hospital’s Brad Bell, RN, was among the first to be vaccinated against covid-19 on Dec. 18 in Ilwaco. The protective shot was administered by Michelle Kemmer, RN.
ILWACO — Hope has come to Pacific County in the battle against covid-19, as Ocean Beach Hospital received and administered the first doses of Pfizer’s coronavirus vaccine to several of the hospital’s employees on Dec. 18.
Trending
The hospital received a shipment of 975 doses of the vaccine on Thursday, Dec. 17, the first doses to arrive in Pacific County. OBH administered the first of the received doses the next day to several of its healthcare workers, including a nurse, physician’s assistant and physical therapist.
As of this Monday afternoon, OBH has administered 80 shots of the vaccine, according to Blair Oman, public information officer for the OBH Covid-19 Incident Command System.
For now, doses in Pacific County — and the country — are going to people who comprise Phase 1a; high-risk health workers and some first responders, as well as staff and residents of long-term care facilities. In conjunction with county health officials, OBH is developing a vaccine distribution plan for how it will administer doses of the vaccine it receives, Oman said.
Trending
The doses at OBH are currently being stored in the container they were shipped in, since the hospital does not have an ultra-cold freezer that the vaccine needs to be stored at to remain viable. Pacific County officials purchased a freezer for OBH, but it isn’t expected to arrive until mid-January due to a backlog with the vendor.
The container storing the doses can be recharged with dry ice five times — it has been recharged once at OBH so far — and the doses of the vaccine being stored in the container should be given within 30 days.
On a daily basis, Oman said, only the doses needed that day can be taken from the container and prepared for use. Once the container has been opened twice, it cannot be opened again on the same day.
Each vial contains enough vaccine for five doses, although healthcare facilities are noticing that there is often enough vaccine for six and sometimes even seven doses.
Another vaccine on the way
On the same day that the first vaccine shots were administered in Pacific County, more good news came as the Food and Drug Administration announced it had approved an emergency use authorization for Moderna’s covid-19 vaccine, following a recommendation the previous day from a panel of health officials and scientists.
The approval gives the country two vaccines in the fight against the virus that has killed more than 320,000 Americans. The Washington State Department of Health said last week that it expects to receive an initial allocation of 128,000 doses of Moderna’s vaccine early this week, and another 55,800 doses next week.
Like Pfizer’s, Moderna’s vaccine calls for a two-dose regimen, to be taken about 28 days apart. Late-stage clinical trials for both of the vaccines suggested the vaccines were at least 90% effective, which Pacific County Public Health Officer Dr. Steven Krager called “awesome.”
Moderna’s vaccine is simpler to store than Pfizer’s, however. Moderna’s vaccine can be stored at 2 to 8 degrees Celsius, about the temperature of a normal refrigerator, for up to 30 days, the company claims. Pfizer’s vaccine can last up to five days when refrigerated. Moderna’s vaccine should be easier to store and distribute for rural areas and communities that don’t have ultra-cold storage freezers that are needed to store Pfizer’s vaccine for an extended period of time.
Next in line
On Dec. 20, a panel advising the Centers for Disease Control and Prevention recommended that the next group of people to receive the vaccine — as Phase 1b — consist of people age 75 and older, along with frontline essential workers such as teachers, food workers, public transit and postal service employees and emergency responders.
The non-binding recommendations — decisions on vaccine prioritization are ultimately made at the state level, although many states have followed the panel’s recommendations so far — would consist of about 49 million people; about 30 million frontline essential workers, and another 19 million people who are 75 and older. For reference, Phase 1a consists of about 24 million people.
Members of the panel concluded at their Sunday meeting that it made sense to include older seniors in the next phase, due to a higher death rate seen in older people than other segments of the population. Earlier in the process, they indicated that they would instead recommend a broader group of Americans defined as essential by the Department of Homeland Security — consisting of about 90 million people.
A vaccine is not expected to be made available to the general public until sometime this spring, at the earliest. Earlier this month, Moncef Slaoui, chief scientific advisor of the U.S.’s Operation Warp Speed, said the U.S. hopes to have vaccinated 100 million people by the end of February, based on the number of doses the country is expected to receive from Moderna and Pfizer’s vaccines.









